NASA's Cassini-Huygens mission detected a large cloud of toxic hydrogen cyanide (HCN) on Titan, one of Saturn's moons. Titan also contains ethane. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom: XeF 6: We place three lone pairs of electrons around each F atom, accounting for 36.. HCN, hydrogen cyanide, is rather poisonous. HCN is a gas used primarily in chemical synthesis, mining, and polymer manufacturing. But serious, it's dangerous, so stay away unless you are a legit chemist. Method 1: Step method to draw the Lewis structure of HCN. In this method, we find the bonds and lone pairs for the whole molecule, then plug.
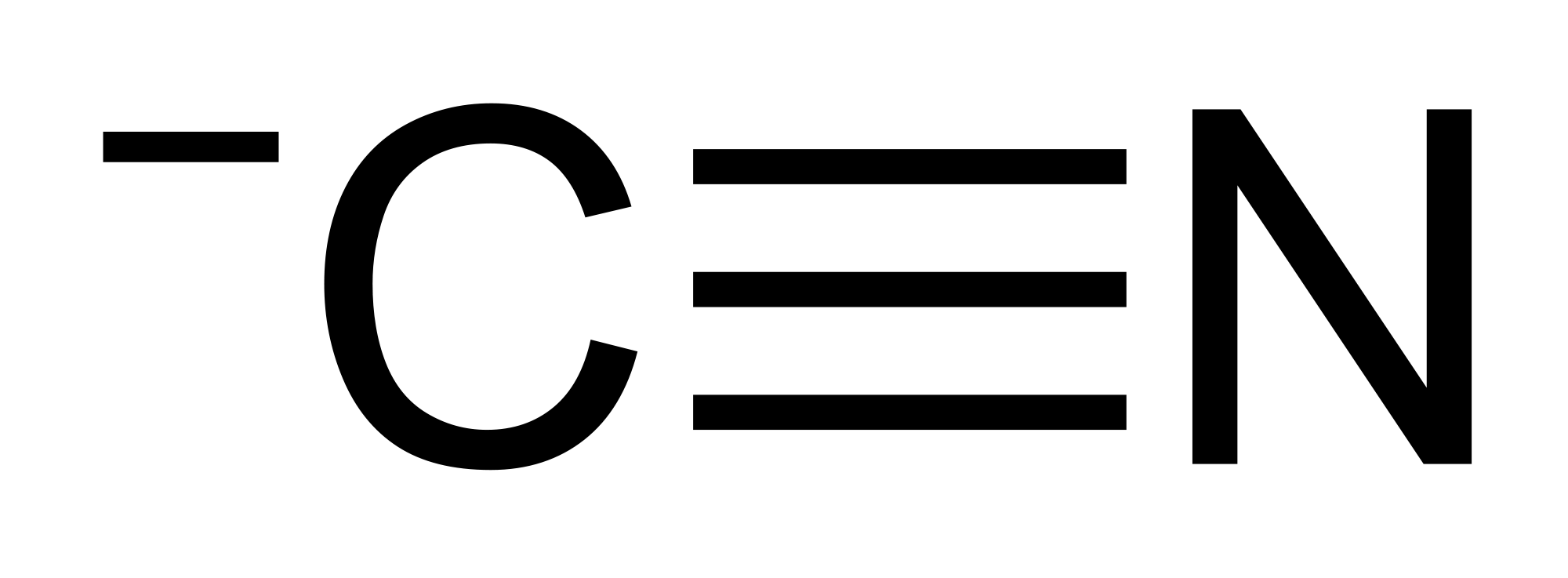
Question 3860d Socratic
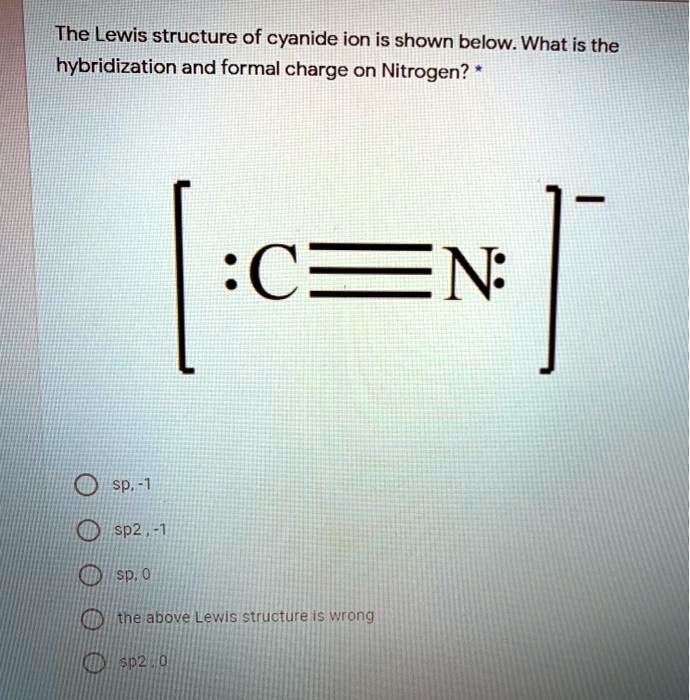
The Lewis structure of cyanide ion is shown below Wh… SolvedLib
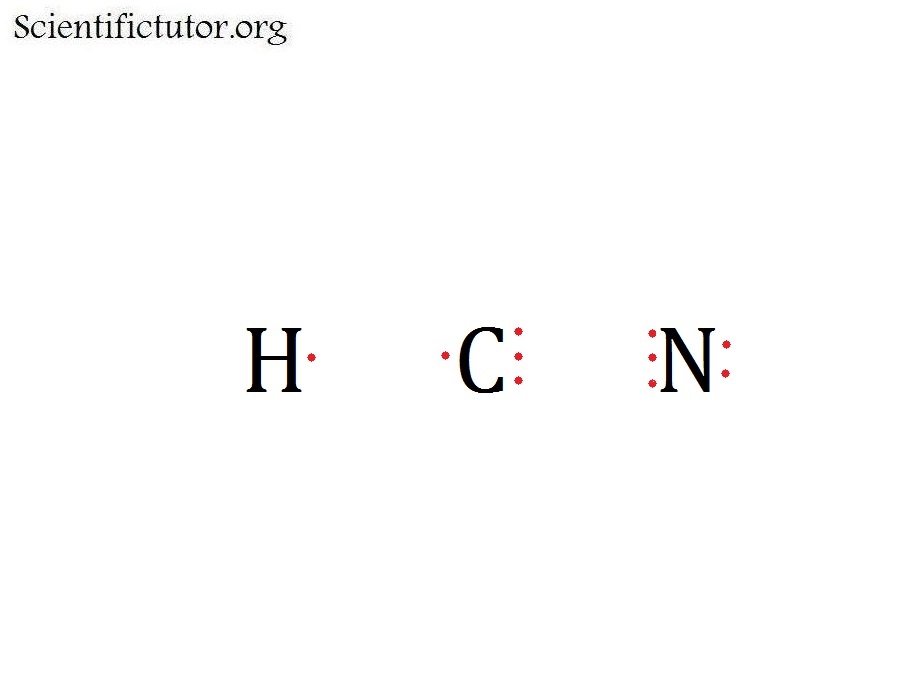
Hcn Dot Diagram Wiring Diagram Pictures

What is the Lewis structure of Hydrogen Sulfate ion (\text{ Quizlet
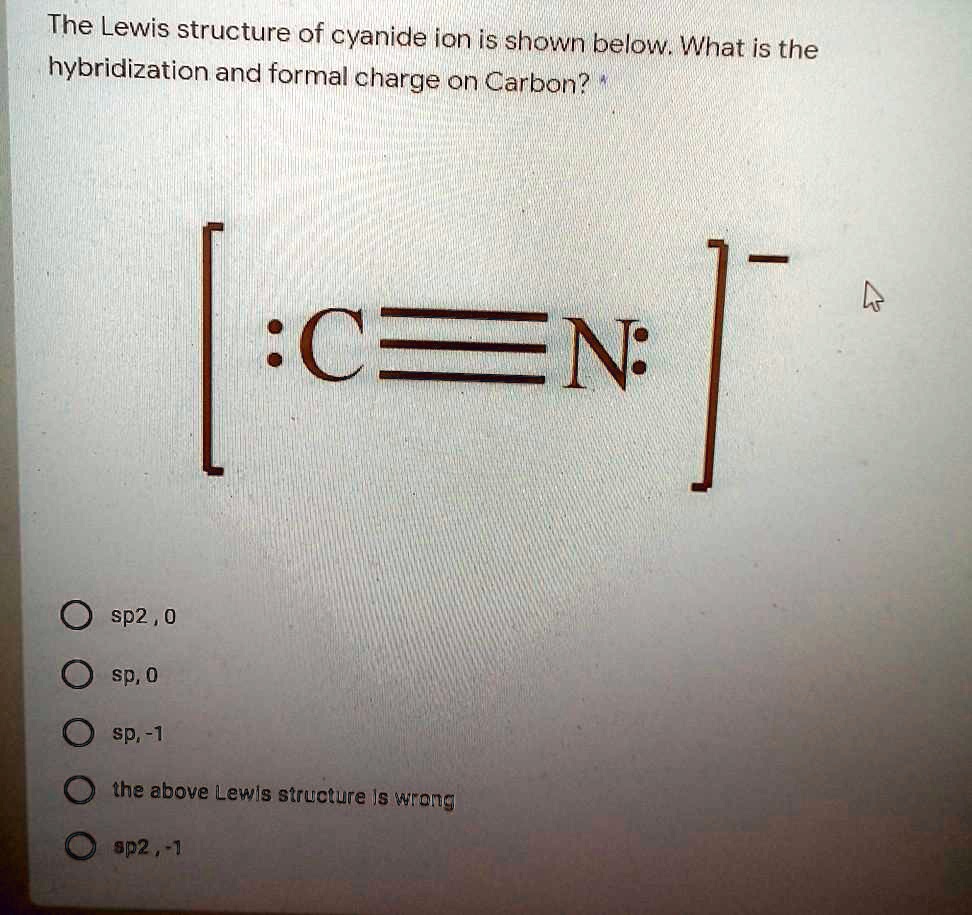
SOLVED The Lewis structure of cyanide ion is shown below. What is the hybridization and formal
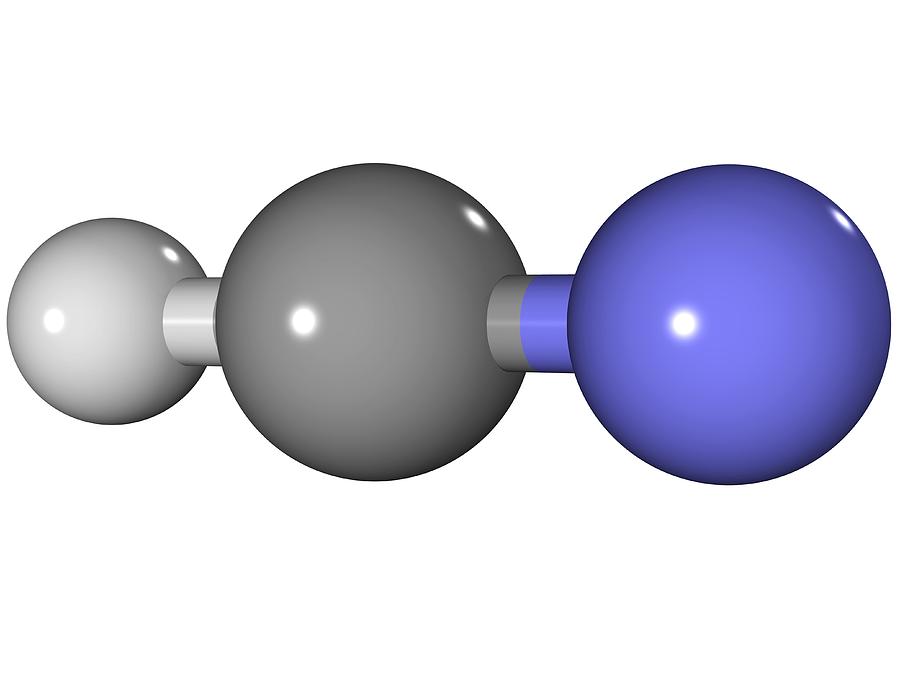
Hydrogen Cyanide Molecule Photograph by Laguna Design Pixels

Hcl Molecule

the chemical symbol for h cen is shown in black and white with an image of

12+ Hydrogen Lewis Dot Structure Robhosking Diagram
[Solved] Draw the Lewis structure for it as well. 3. Hydrogen cyanide (HCN)... Course Hero
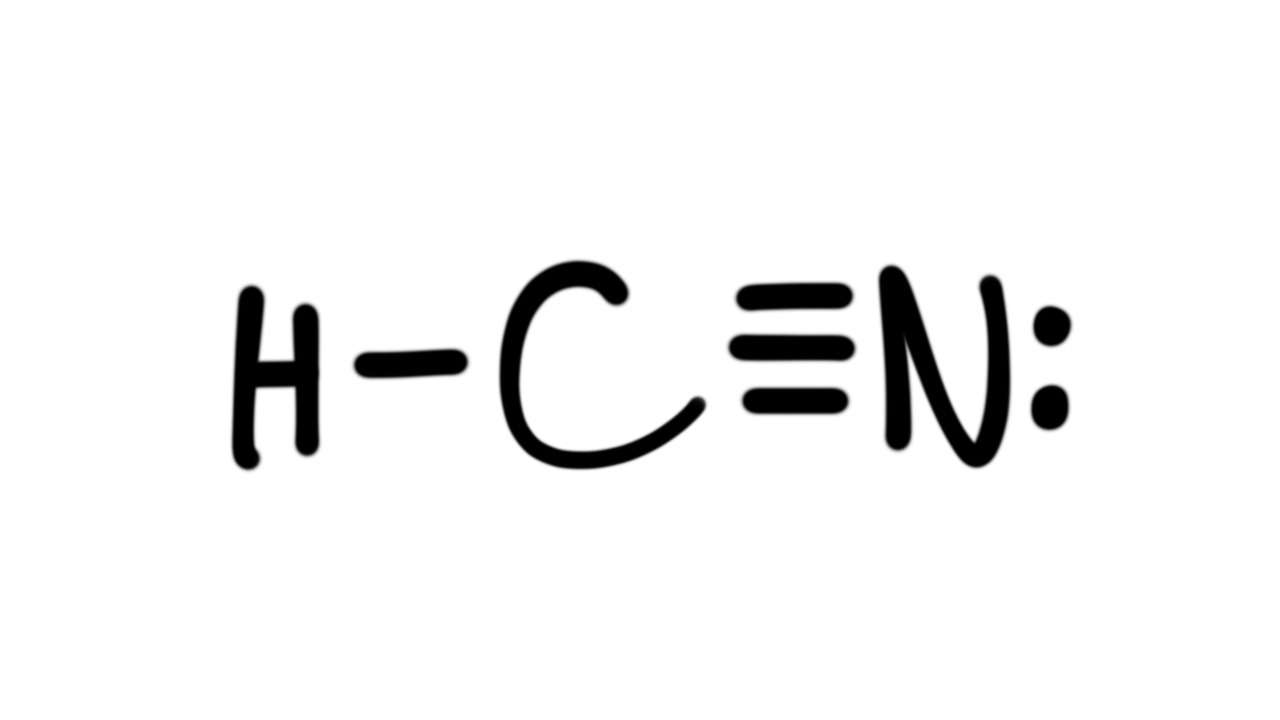
Hydrogen Cyanide YouTube

Hydrogen Cyanide Lewis Structure

Hydrogen Cyanide
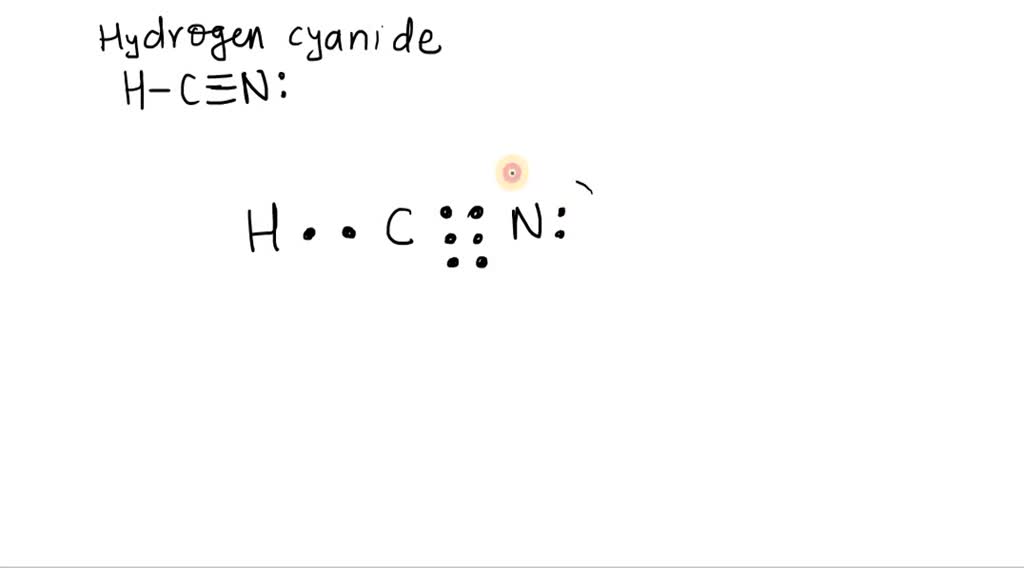
SOLVEDThe Lewis structure for hydrogen cyanide is HC ≡N Draw circles enclosing electrons to
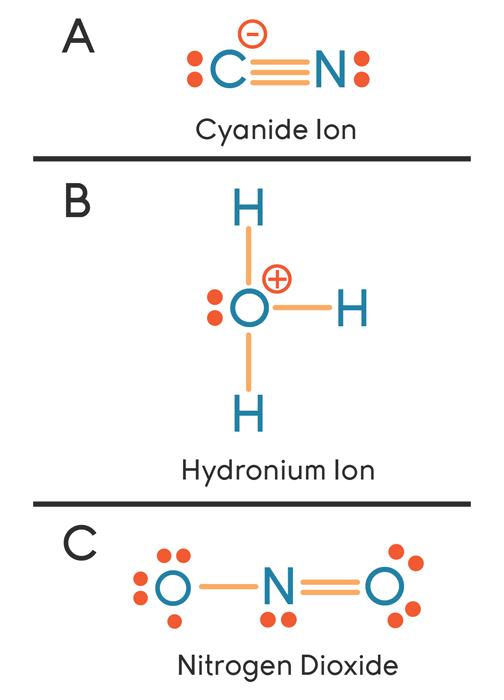
Cyanide Ion Lewis Structure
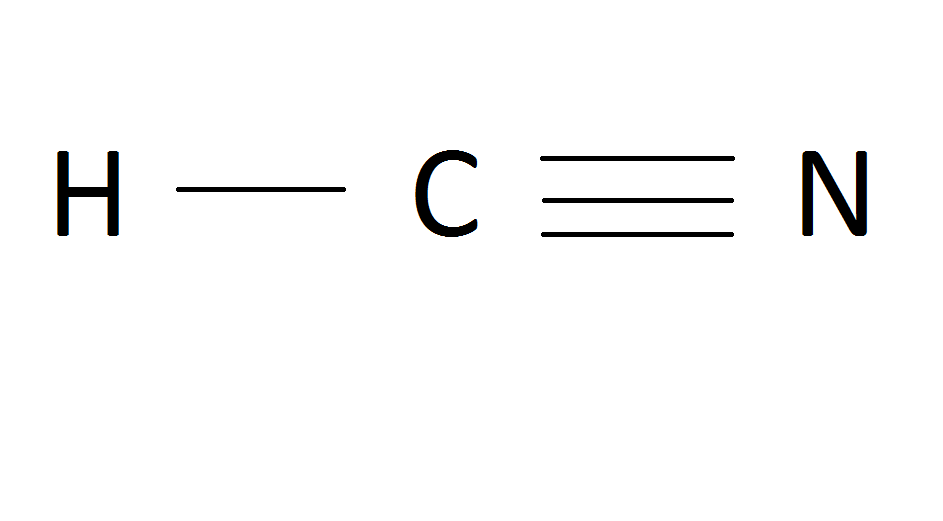
2P3 LSS February 2011

Draw the Lewis dot structure of Hydrogen cyanide (HCN) molecule
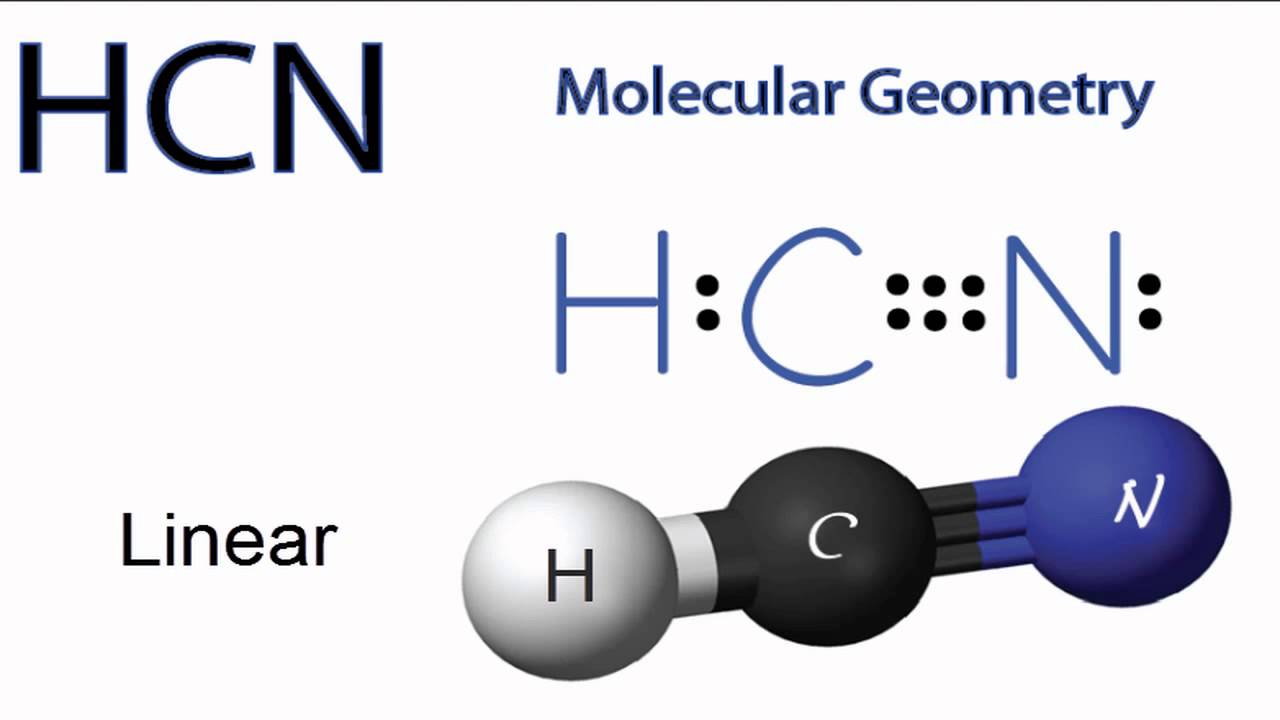
HCN Molecular Geometry YouTube

HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and Polarity Techiescientist
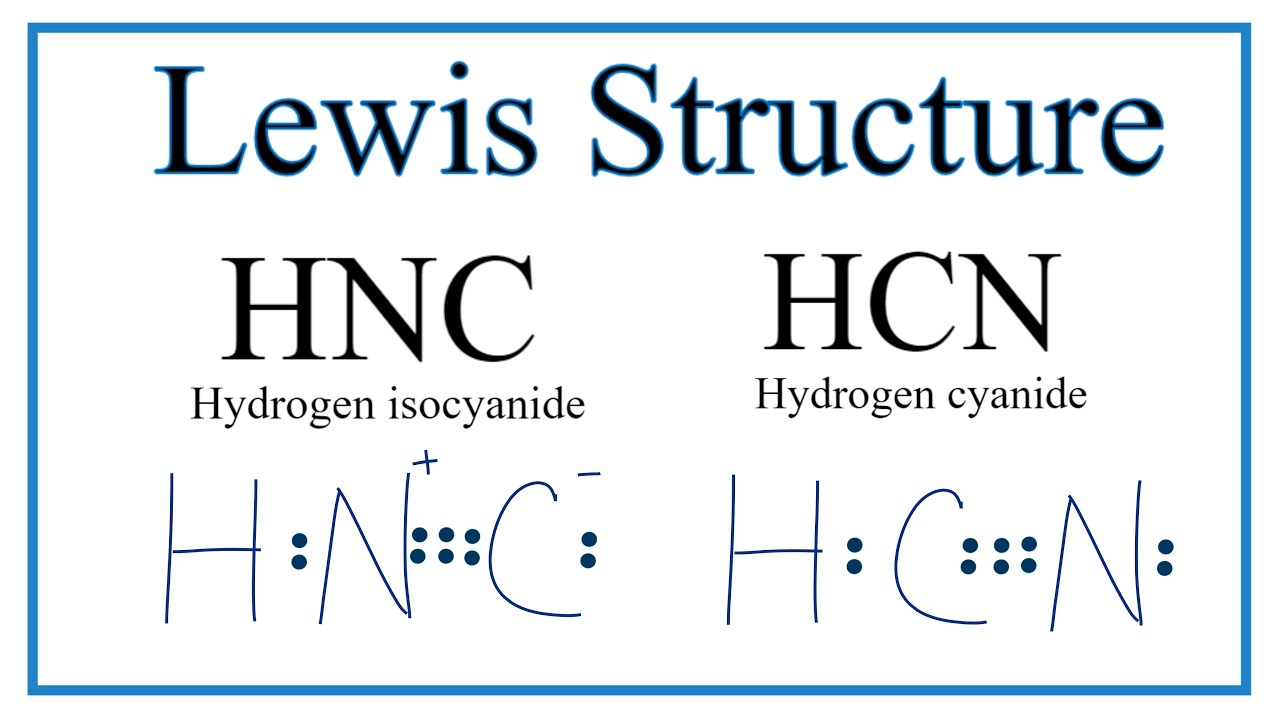
Hcn Lewis Structure
Draw the Lewis structure for the hydrogen cyanide (HCN) molecule. Your solution's ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on.. Drawing the Lewis Structure for HCN. Make sure you put the correct atom at the center of the HCN molecule. With the Lewis Structure for HCN you'll need to share more than one pair of electrons between the Carbon and the Nitrogen atoms. Be sure that you don't use more than the ten valence electrons available.